Blog
Challenges When Testing for Enterobacteriaceae

This blog post is a 23 minute read
In the food and beverage industry, there are few things as important to quality and reputation as microbial safety.
The Centers for Disease Control and Prevention (CDC) estimates that each year, around 9.4 million Americans will get sick from a foodborne illness. Studies that have examined the cost of recalls estimate that the average cost of a food product recall in the U.S. is $10 million—and there are hundreds of recalls every year.
With this in mind, there is clearly a lot to be considered when selecting the right microbial indicator test and method of sampling.


Let’s take a close look at what indicator testing is, some of the challenges of conducting testing, and what factors are important to consider when picking the right test to meet specific situational requirements. In this example, we’re looking at the situation requirements of the poultry industry and why Enterobacteriaceae is used as one of the indicator microorganisms in that industry.
What is Indicator Testing?
Indicator testing detects generic strains of bacteria, as opposed to testing for specific organisms or pathogens. Enterobacteriaceae are a large family of different types of bacteria containing generic coliform and E. coli subgroups. Enterobacteriaceae (EB) are commonly found in fecal material, which nobody wants in their food.
Many EB are harmless, but may be more prevalent in certain industries, such as poultry, and are closely related to pathogens frequently named in the news during outbreaks. Some of these Enterobacteriaceae pathogens are Salmonella, Shigella, Yersinia, and hemorrhagic E. Coli.
Indicator testing is useful for monitoring broad microbial trends of commonly found and easily tested microbes, like Enterobacteriaceae, rather than hunting for exact matches of less frequently found and harder to detect pathogenic bacteria. Because pathogenic bacteria can be hard to detect, poultry indicator testing, such as Enterobacteriaceae, broadly looks to identify the relatives to pathogens, such as Salmonella, that may be present in the food production process.
This is beneficial for two reasons. First, many kinds of pathogenic bacteria typically exist in the environment at low levels, making them difficult to find and testing for these pathogens can be very expensive.
And second, because this kind of testing detects more common and prevalent generic strains, it can be an important bellwether showing that the right conditions exist for pathogenic bacteria to flourish. Indicators are common bacteria that serve as indirect warnings that more dangerous bacteria are likely to be present.
This allows for proactive remediation and cleaning of surfaces on the indicator result, rather than the long wait, where surfaces can be potentially harboring harmful bacteria, potential product hold or plant shutdown, and detailed notification/follow-up testing that has to occur to verify the existence of a pathogen.
Simply stated, indicator testing is like a warning light blinking on your dashboard—it gets your attention and tells you some kind of mitigation is necessary.
Testing Challenges
Traditional methods of bacterial indicator testing require preparation time and proficient lab skills. Unfortunately, due to a lack of microbiological expertise in food plants, samples are frequently sent out to an offsite third-party lab for culturing and results. This is almost always a more time-consuming and expensive route than onsite testing, whether that means using the standard method pour plates or an alternative, ready-to-use method.
There are many different onsite testing methods available, but how do you know which one is right for your facility? Even some of the faster, ready-to-use microbial test options available for onsite testing can be problematic, mostly when trying to determine their results.
Some require the use of a spreader device, which can cause the test sample to spread outside of the testing area, even when performed by an experienced technician. When this occurs, the test is invalid and requires time-consuming (and expensive) retesting. When interpreting results, methods requiring gas and acid halo-identification can provide ambiguous, hard-to-read results. This necessitates significant in-house expertise to conduct, read, and interpret tests because this isn’t a time for guesswork.
Conducting Enterobacteriaceae indicator testing with Peel Plate® EB Microbial Tests is faster than traditional methods and older, ready-to use tests, allowing for speedier remediation and less downtime. Peel Plate® tests were specifically designed to be easy to use and easy to interpret by using color indicators.
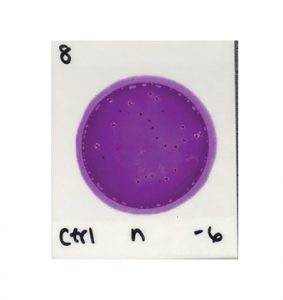
For example, to read the results in the image below you must determine which colonies are associated with gas bubbles and/or yellow halos to identify the EB colonies. This differentiation requires training and expertise.
3M™ Petrifilm™ Enterobacteriaceae Count Plates
In contrast, to determine the results in the next image, all you need to do is count the colonies.

Charm Peel Plate® EB Microbial Test
The second test is easier to prepare, due to a self-wicking medium that doesn’t require a spreader device. It is also easier to read and interpret—just count all of the colonies that show up on the test, regardless of size, color, gas production, etc.
Poultry Industry Testing
A recent CDC report confirmed that chicken was the most frequent cause of foodborne illness outbreaks in the U.S., comprising 12% of the confirmed cases. While only a small percentage of the reported illnesses in the U.S. are connected to recognized outbreaks, poultry safety is a top priority for manufacturers and government agencies.
The poultry processing industry is under constant scrutiny from regulatory bodies in the United States. USDA-FSIS inspectors work onsite at processing plants conducting daily inspections and pulling product samples for microbial (pathogen) testing. Concurrently, plants are required to self-monitor their manufacturing and sanitation processes by performing their own additional indicator and pathogen testing. Plants are allowed to use their data to show due diligence in their microbial control and sanitation efforts.


Plants are scored by regulatory agencies on a scale of 1-3, based on pathogen test results:
- Facilities with a score of 1 are considered in microbiologic control and have “achieved 50 percent or less of the maximum allowable percent positive during the most recent completed 52-week moving window.”
- Facilities with a score of 2 are considered marginally in control and have met “the maximum allowable percent positive but have results greater than 50 percent of the maximum allowable percent positive during the most recent completed 52-week moving window.”
- Facilities with a score of 3 need some improvement and have “exceeded the maximum allowable percent positive during the most recent completed 52-week moving window.”
In short, a score of 1 is good, and a score of 3 is problematic.
FSIS inspectors use buffered peptone water (BPW) to conduct tests of raw poultry. BPW is essentially a sampling medium that helps food safety inspectors recover Salmonella and other pathogenic bacteria in raw poultry. Many of the existing indicator tests for poultry on the market are not validated specifically for poultry testing.

The industry needs a simple-to-use, affordable test method that provides accurate and timely results when testing a variety of food and environmental matrices.
Charm Peel Plate EB Microbial Tests
Charm’s microbial test addresses many of the common challenges of Enterobacteriaceae testing. In addition, this test has been validated by OMA for specific industry uses, including poultry carcass rinse testing in BPW.
OMA (the Official Methods of Analysis program of AOAC International) validation is recognized worldwide, something ever-important in today’s global marketplace.
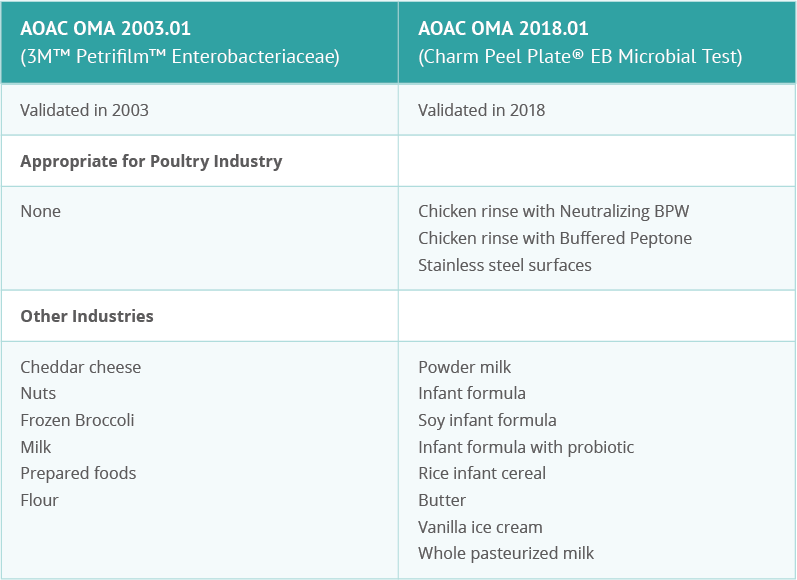
Charm’s Peel Plate EB Microbial Test is an easy to use, easy to understand test for Enterobacteriaceae that produces fast results (24 hours). And, it’s approved specifically for poultry testing with BPW, which is used by most processors.
With the availability of a validated indicator method for Enterobacteriaceae in poultry rinse with BPW, industry members can take steps towards aligning their sampling methods with the USDA-FSIS method and performing their required testing with just a single test sample. This sample can be used for both indicator testing and required pathogen testing and meets the FSIS sampling requirements.
Not only are the tests easier to interpret and faster to perform than other indicator test methods, they also comply with FSIS sampling requirements. The tests are easier to justify and defend during audits and inspections.
This sample can be used for both indicator testing and required pathogen testing and meets the FSIS sampling requirements.
Don’t Just Take Our Word for It…
We’re of course proud of the Peel Plate EB Microbial Test, but what matters most is how the product works for our customers. Here’s what one customer had to say about the Peel Plate EB test:
“Charm Peel Plates have provided exceptional benefits to our manufacturing plant. The ease of use was a must have for us. We were able to teach plant operators, who have no lab background, how to dilute and properly plate, incubate, and read samples without having to hire additional staff.
Prior to implementing the program, there was a lot of lead time waiting on results and hoping what we made was good. We would send out 5-6 samples to the lab for a single lot of product. Now, we can plate our samples, wait the short period for the results, and package or immediately implement corrective actions. We are down to sending one sample out per lot as a formality and confirmation of results. The plates have saved us significant time and money.”
Using Charm’s Products to Keep Your Facility Safe
Microbial indicator tests are an important low-cost and simple approach used by the food industry to verify their sanitation control programs and reduce customer risk spoilage and disease by monitoring microbial levels. The poultry industry is an excellent example of a proactive food supplier that performs these tests, choosing EB as an indicator because of its relationship to a Salmonella risk.
But there remain cost and performance challenges that need solutions. Charm Sciences is helping the food industry address those challenges with low cost, simple-to-use tests and sampling methods that are validated for their intended purpose. Saving our customers time and money while protecting their brand with accurate, easy-to-use tests is what Charm Sciences work towards every day.

If you’re in the poultry processing or food industry and want to try Charm Peel Plate EB Microbial Tests, please contact us. We’ll work with you to select the right combination for a free trial.
About Charm Sciences
Established in 1978 in Greater Boston, Charm Sciences helps protect consumers, manufacturers, and global brands from a variety of issues through the development of food safety, water quality, and environmental diagnostics tests and equipment. Selling directly and through its network of distributors, Charm’s products serve the dairy, feed and grain, food and beverage, water, healthcare, environmental, and industrial markets in more than 100 countries around the globe. https://www.charm.com
